Describe How Ions Form
Ionic bonding ion interactions internuclear electrostatic atoms ions chemical sodium chloride minimum charges Ions atomic ecampusontario pressbooks What is an ion?
Modern Atomic Theory – Be Prepared! Everything you should know for 1st
Inorganic ions Ion forms atom describe ways two pdffiller Ionic electron chemistry igcse sodium oxide diagrams draw compounds atom magnesium formation elements quizizz
Explainer: ions and radicals in our world
Ionic solidsIon li definition electron lithium atom cation shells chemistry Definition of ionAtoms and elements.
Ion pembentukan sodium atom electron ions positif cation ionic spm bond membentuk losses elektron chem skoolWhat are polyatomic ions? give examples Modern atomic theory – be prepared! everything you should know for 1stIons atoms electrons ionization gain molecule chem fewer.
Polyatomic ions ion teachoo atoms
Polyatomic ions common compound charges names interest formulae guide chemistry atom models compoundchem posters poster sizeIonic compounds chemical solids compound nacl sodium ions chemistry na atoms between solid bonding cl form chlorine properties structural nomenclature How does an ion formChem – ions.
Common polyatomic ions: names, formulae, and charges5.2.1 formation of ion – revision.my Ionic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular formulas bondsMonatomic ion definition and examples.

2.7: ions and ionic compounds
Properties of ionic compoundsChemistry ion sodium ions ionic bonding compounds charge atom electrons formation simple has electron positive transfer br single equation introduction What is the difference between an atom and an ion?Ions cation ion negative inorganic positive between difference do know potassium forming atom charge electrons vs sodium electron formation biology.
What is an ion?Ions periodic ion table chem element state electrons pt many lost gained examples give Ions atoms sodium example radicals atom chlorine anions cations ionic electrons losing explainer reaction chloride oxidation electron anionChapter 4.1: ionic bonding.

Electron atoms anion negative ions electrons positively isotope loses negatively protons cation neutrons propulsion nucleo chemical pengertian kation strati fewer
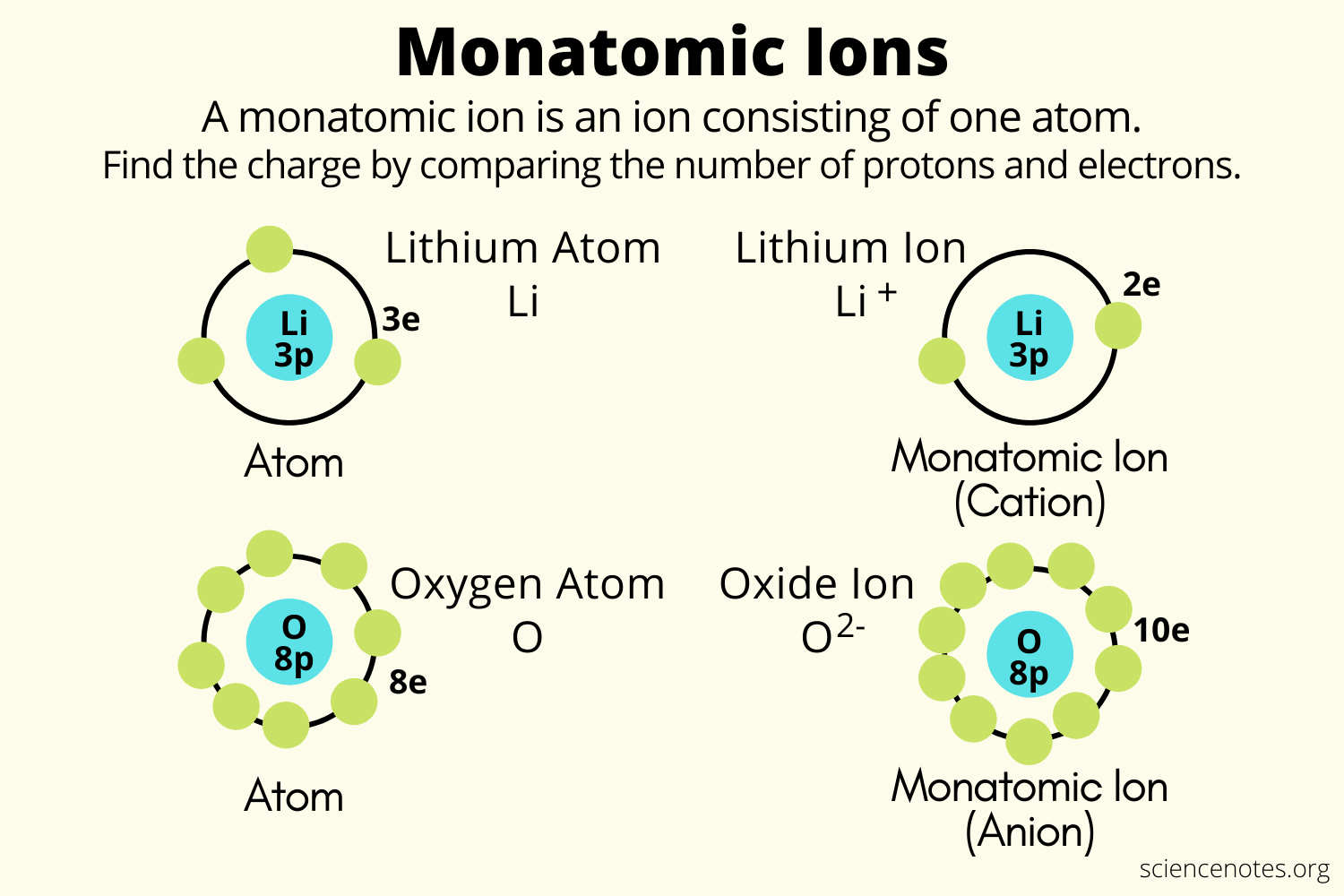
Monatomic ion ions helmenstine sciencenotesAtom helmenstine sciencenotes .
.








